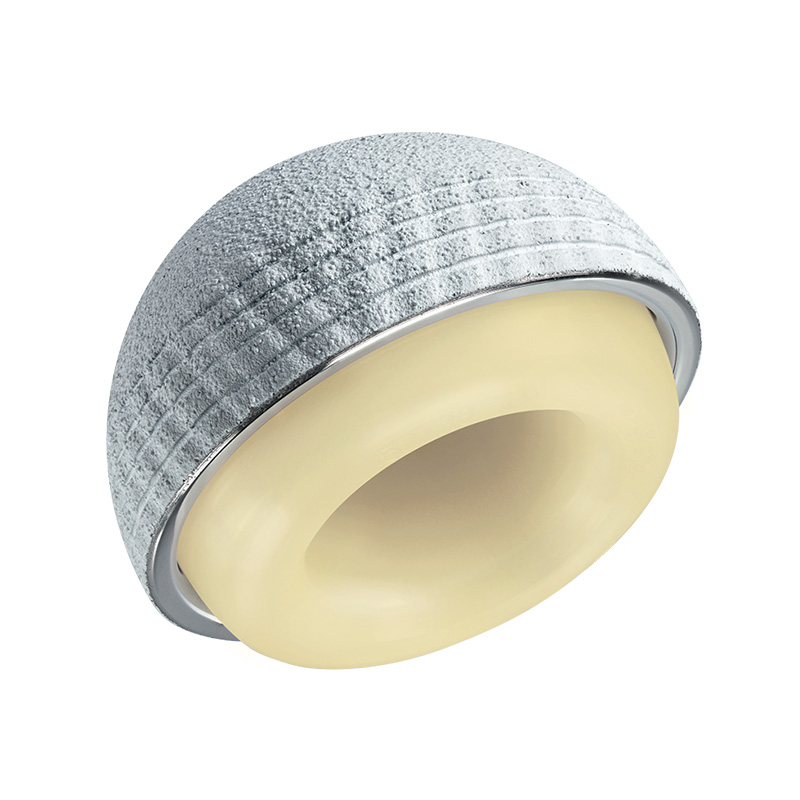
The Monoblock Dual Mobility Concept
Dual Mobility cups were introduced in 1974 by French surgeon Professor G. Bousquet. The original Monoblock design limited the risk of postoperative instability and dislocation along with reducing friction and wear.
For the past 50 years since then, Monoblock cups have continued to improve stability, safety and prosthetic survival rates for patients throughout the world.
Monoblock cups also continue to outperform the more recent Modular dual mobility cup designs which were introduced in 2011, bringing with them the risk of corrosion and cobalt release due to the addition of a metal liner.5
Monoblock Dual Mobility Motion
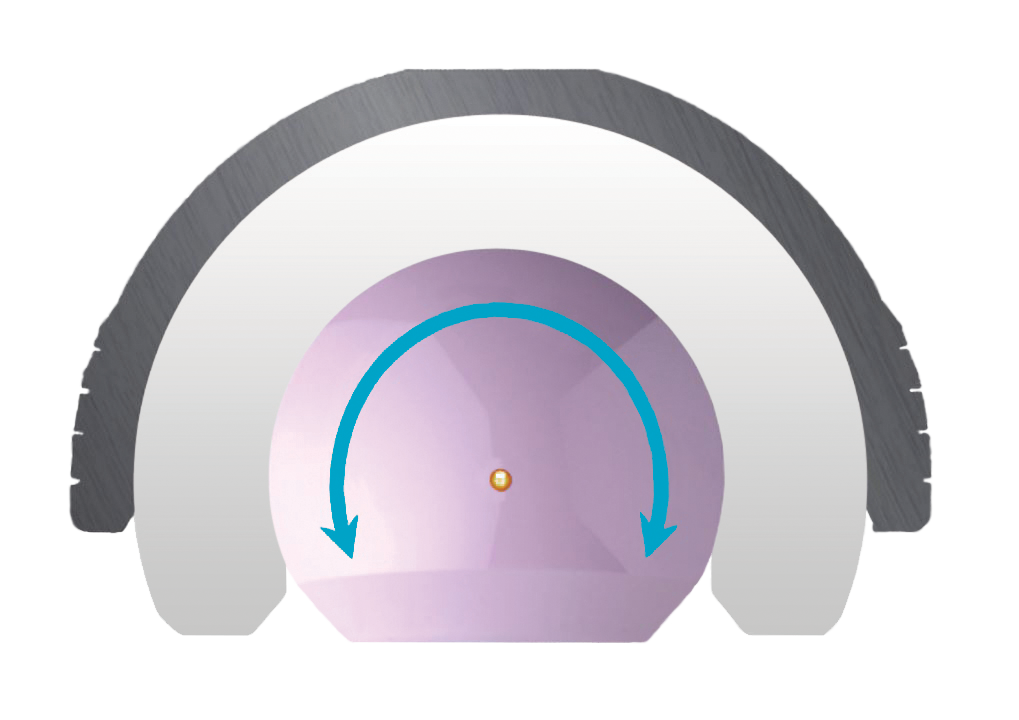
The Monoblock Dual Mobility concept features two sequences of motion:
First Motion
Femoral head and the inner surface of the liner
The head has a small diameter (22.2 or 28mm), allowing a low wear rate due
to less surface friction. This first motion occurs systematically, until the femoral
neck contacts the liner.
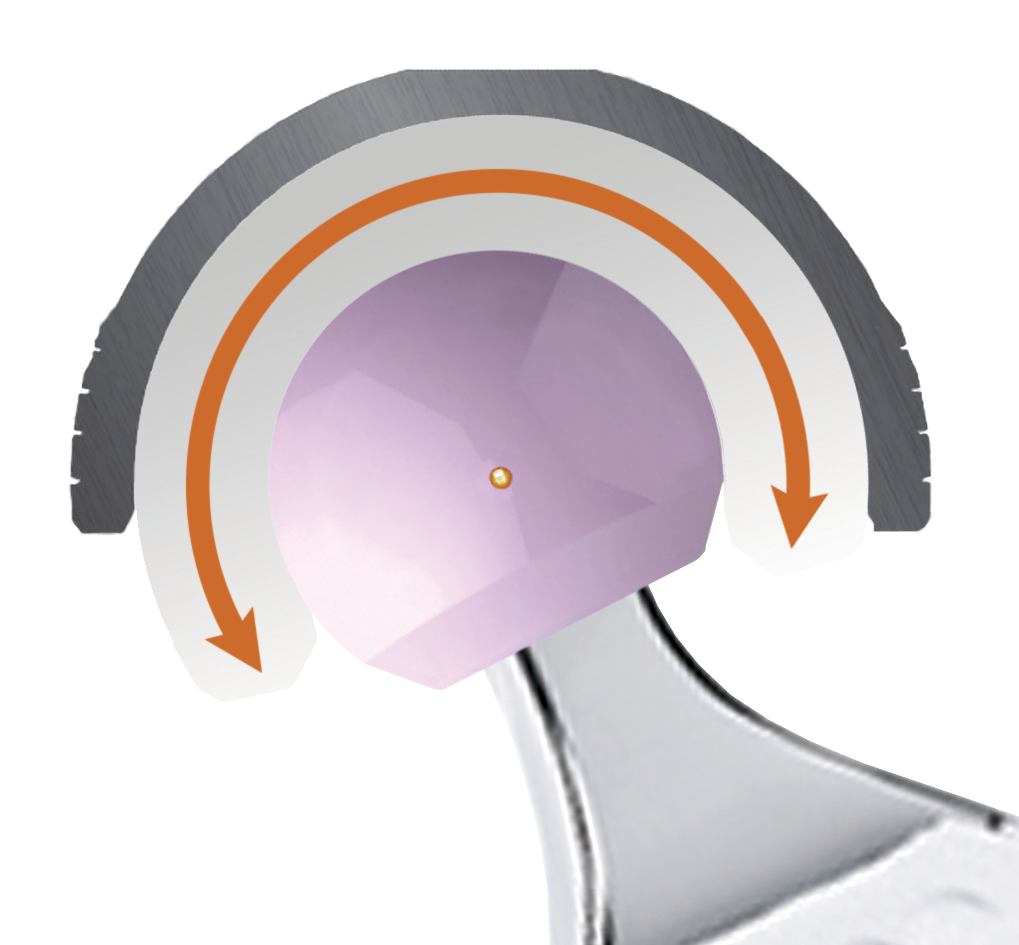
Second Motion
Outer surface of the liner and the inner surface of acetabular cup
The liner acts as a large femoral head articulating in the cup. This second articulation only occurs when a larger range of motion is required. The large liner’s diameter induces a high jump-distance, resulting in superior joint stability.
The Benefits of Monoblock Design
Increased Stability
Dual Mobility cups are clinically proven to reduce the risk of dislocation through broad articulation with the liner.4 Monoblock Dual Mobility cups provide the greatest stability due to the absence of a Modular metal liner which enables a larger poly to be used in the same cup size. This significantly increases jump distance which further reduces the risk of dislocation.

No Cobalt Release
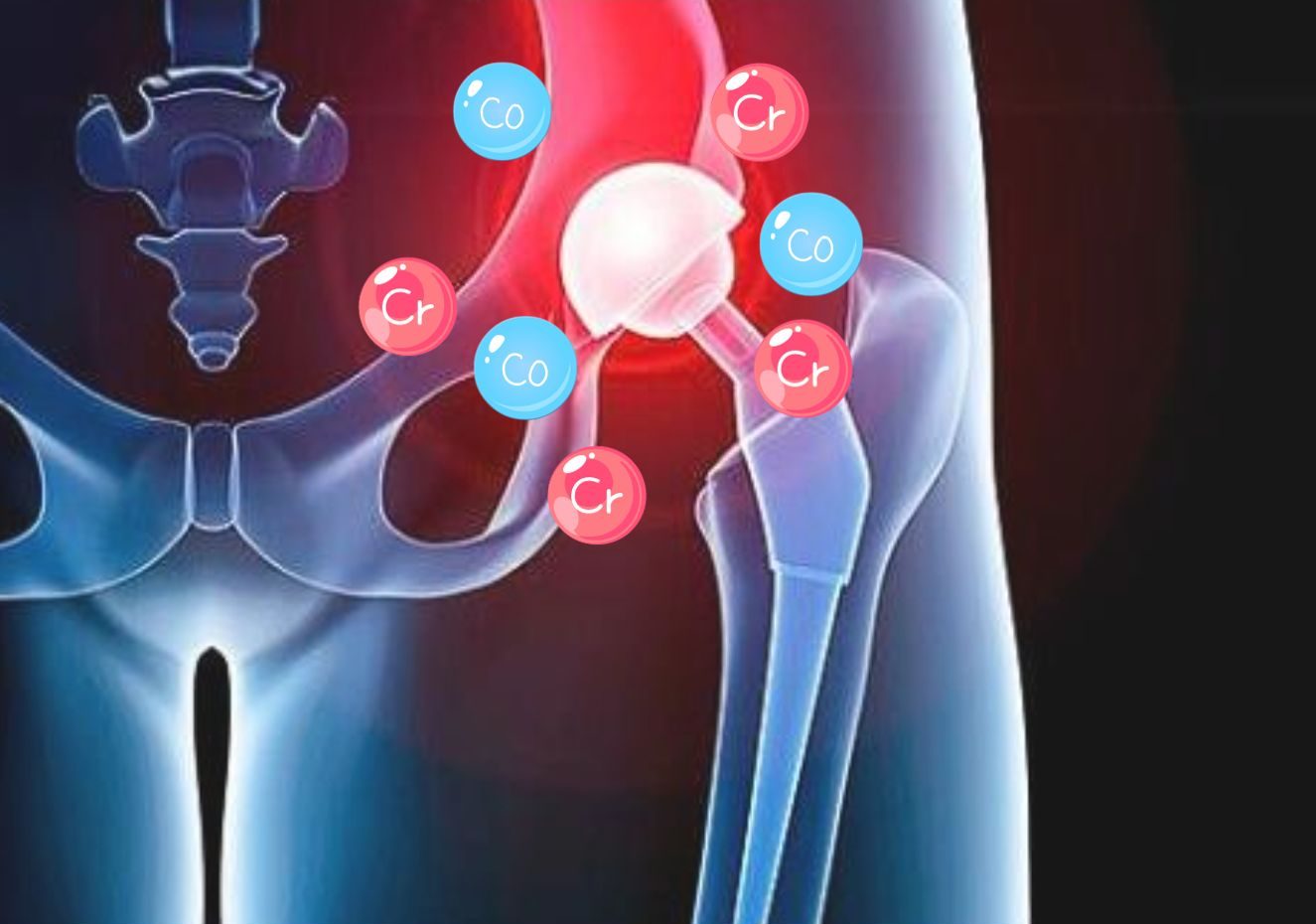
Unlike Modular Dual Mobility designs, Monoblock cups have no metal liner/shell interface which eliminates the risk of metal ion release into the surrounding tissues.3
A 2015 study of 100 consecutive patients implanted with Modular Dual Mobility components found 25% had elevated serum cobalt levels and 9% of those were significantly above normal11.
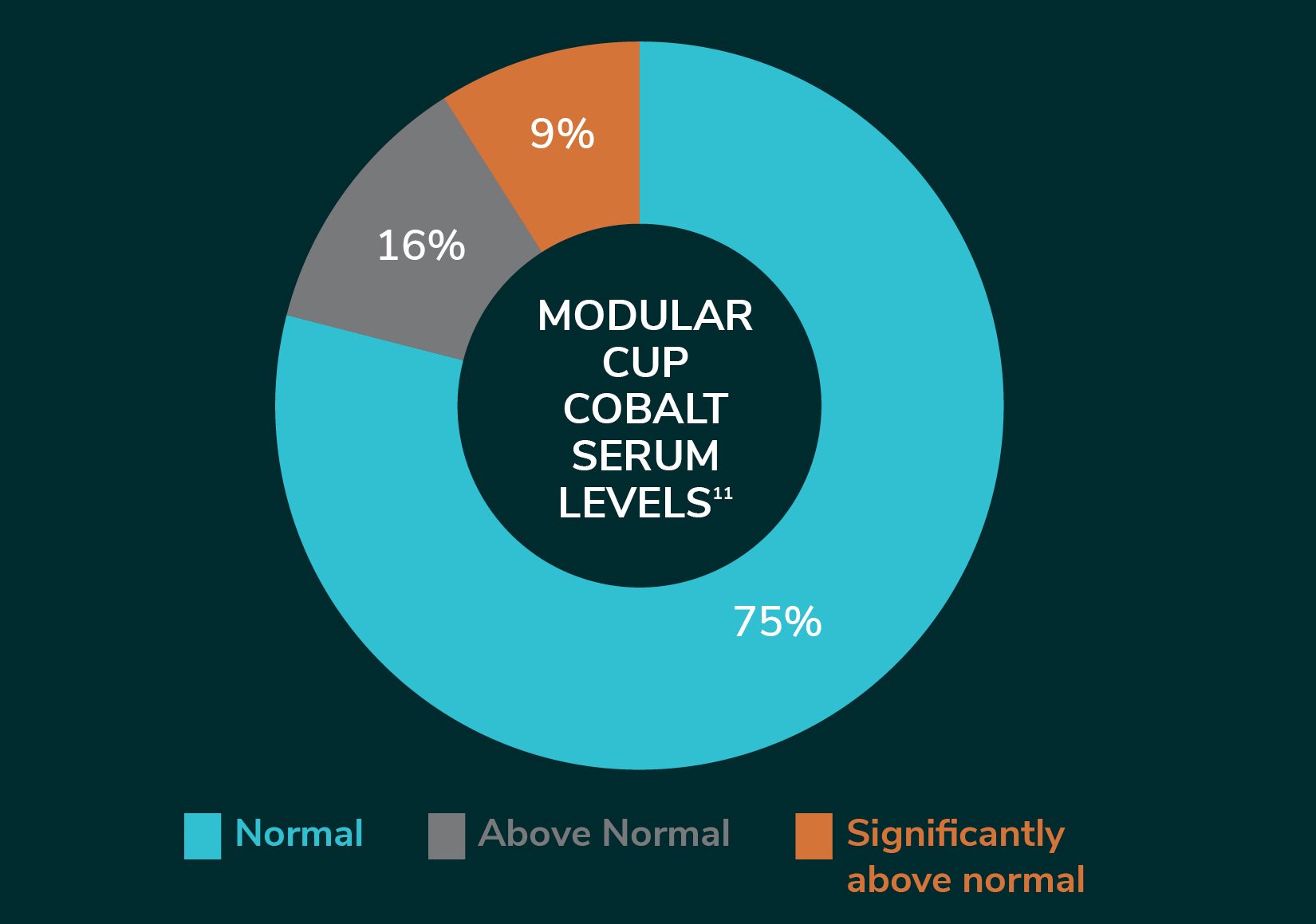
Reduced Corrosion & Wear
A 2020 retrieval study of Modular Dual Mobility metal tapers found all tapers to have noticeable levels of corrosion/wear, indicative of metal debris being lost in the patient.3 Adverse Reaction to Metal Debris (ARMD) encompasses metallosis and ALTR and is a rare but complicated occurrence in THR. The incidence of ARMD in THR performed with Modular Dual Mobility cups has been estimated at 0.3% which is almost ten times higher the 0.032% reported in all non-metal-on-metal primary hip replacements.12

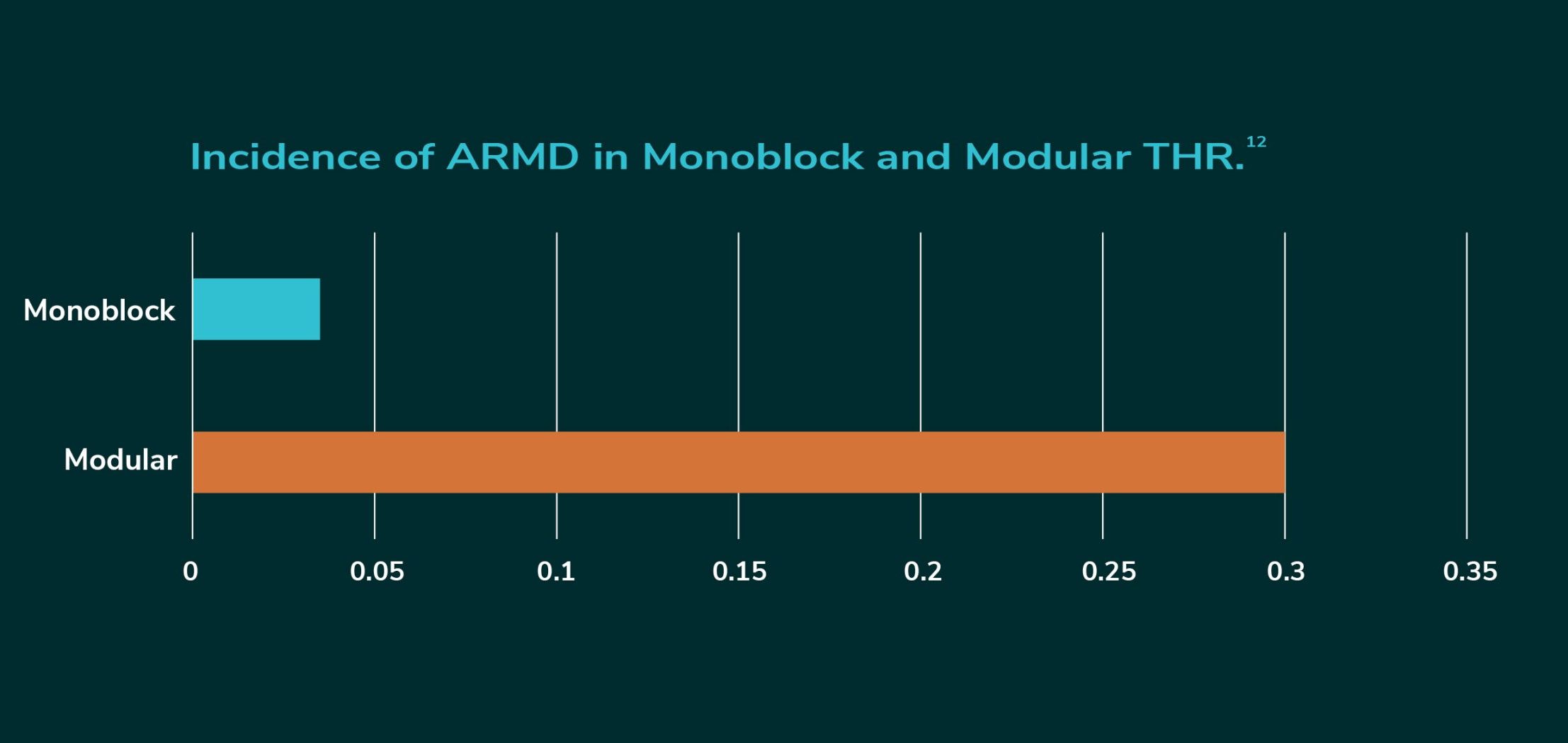
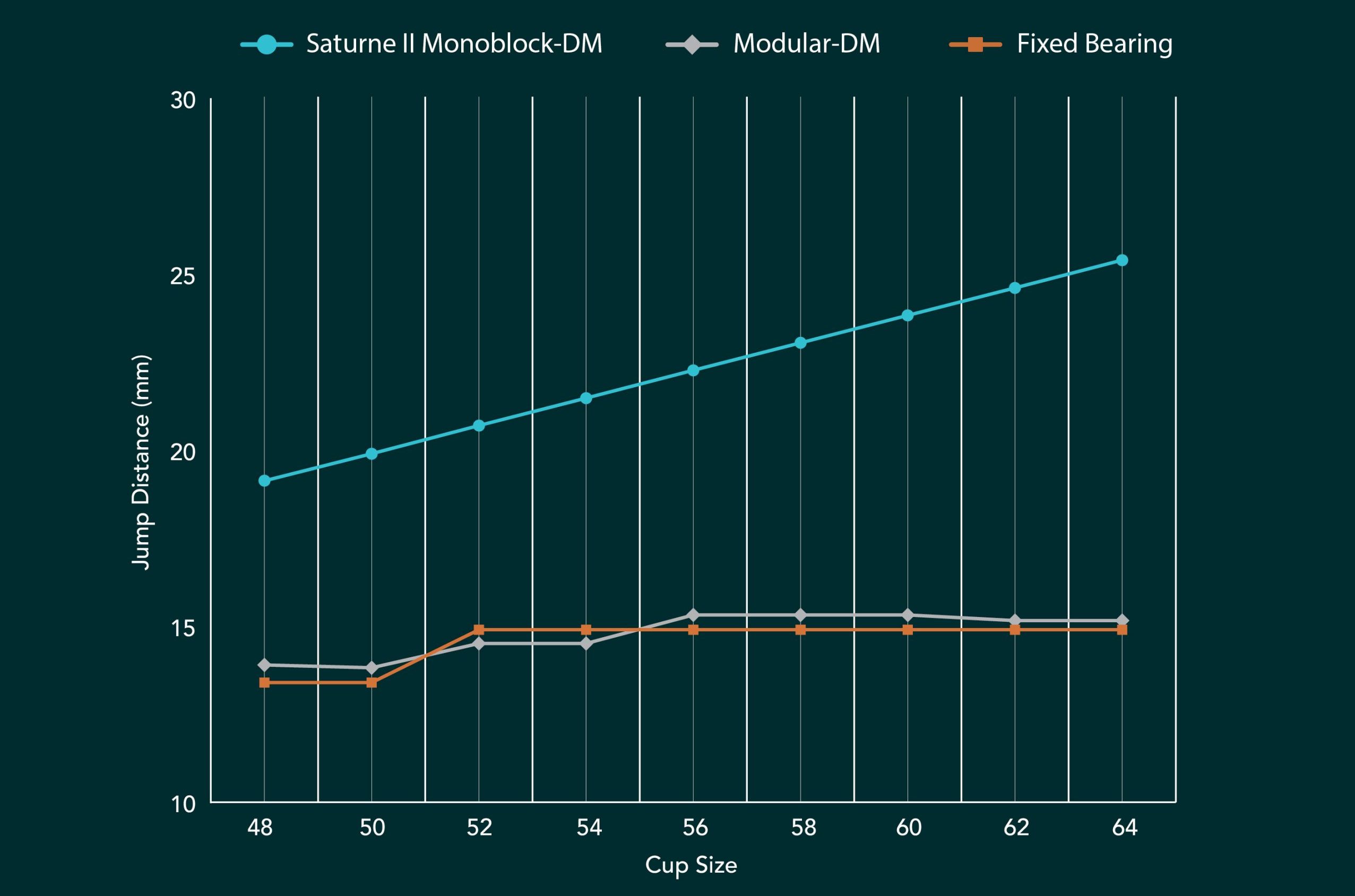
It’s all about Jump Distance
Prosthesis instability and/or dislocation is the second highest cause for revision in primary THR at 22% after infection13. Due to their larger head/liner size, Dual Mobility (DM) constructs have been favoured over fixed bearing cups for patients with a higher risk of dislocation, but it is the Monoblock DM design that results in far greater stability than Modular DM designs due to its significant increase in Jump Distance (JD).
The Jump Distance is the lateral translation required from the femoral head prior to dislocation. The smaller the JD, the higher the theoretical risk of dislocation. In Modular DM designs, the presence of a metal liner alters the centre of rotation, limiting the jump distance. Modular DM designs therefore show only a marginal increase in jump distance when compared to some fixed bearing cup sizes and have a lower JD than fixed bearing cups in other cup sizes. By comparison, the saturne® II Monoblock DM cup shows a significant JD increase over both fixed and modular designs in all cup sizes.4
Jump Distance Comparison
Jump Distance Calculator
References
1. DHR, et Kompetencecenter for Klinisk Kvalitet og Biostatistik, Nord. 2014. DanskHipRegister2014.
2. https://www.odep.org.uk/about/rating-system/
3. Joshua M. Kolz, Cody C. Wyles, Douglas W. Van Citters, Ryan M. Chapman, Robert T. Trousdale, Daniel J. Berry, In Vivo Corrosion of Modular Dual-Mobility Implants: A Retrieval Study, The Journal of Arthroplasty, Volume 35, Issue 11, 2020, Pages 3326-3329, ISSN 0883-5403, https://doi.org/10.1016/j.arth.2020.05.075.
4. Tigani, D., Banci, L., Valtorta, R. et al. Hip stability parameters with Dual Mobility, Modular Dual Mobility and fixed bearing in total hip arthroplasty: an analytical evaluation. BMC Musculoskelet Disord 23, 373 (2022). https://doi.org/10.1186/s12891-022-05280-2
5. AOANJRR Annual Report 2022, Table HT55.
6. AOANJRR Annual Report 2022, pg. 117.
7. AOANJRR Annual Report 2022, pg.118 Table HT56.
8. AOANJRR Annual Report 2022. pg.120 Table HT58.9.
9. Queally JM, Devitt BM, Butler JS, Malizia AP, Murray D, Doran PP, O’Byrne JM. Cobalt ions induce chemokine secretion in primary human osteoblasts. J Orthop Res. 2009 Jul;27(7):
855-64. doi: 10.1002/jor.20837. PMID: 19132727.
10. All metal-on-metal (MoM) hip replacements: updated advice for follow-up of patients (MDA/2017/2018).
11. Matsen Ko LJ, Pollag KE, Yoo JY, Sharkey PF. Serum Metal Ion Levels Following Total Hip Arthroplasty With Modular Dual Mobility Components. J Arthroplasty. 2016 Jan;31(1):186-9. doi: 10.1016/j.arth.2015.07.035. Epub 2015 Jul 23. PMID: 26318084.
12. French JMR, Bramley P, Scattergood S, Sandiford NA. Adverse reaction to metal debris due to fretting corrosion between the acetabular components of Modular dual-mobility constructs in total hip replacement: a systematic review and meta-analysis. EFORT Open Rev. 2021 May 4;6(5):343-353. doi: 10.1302/2058- 5241.6.200146. PMID: 34150328; PMCID: PMC8183148.
13. AOANJRR Annual Report 2022. pg.62 Table HT15
Monoblock Range
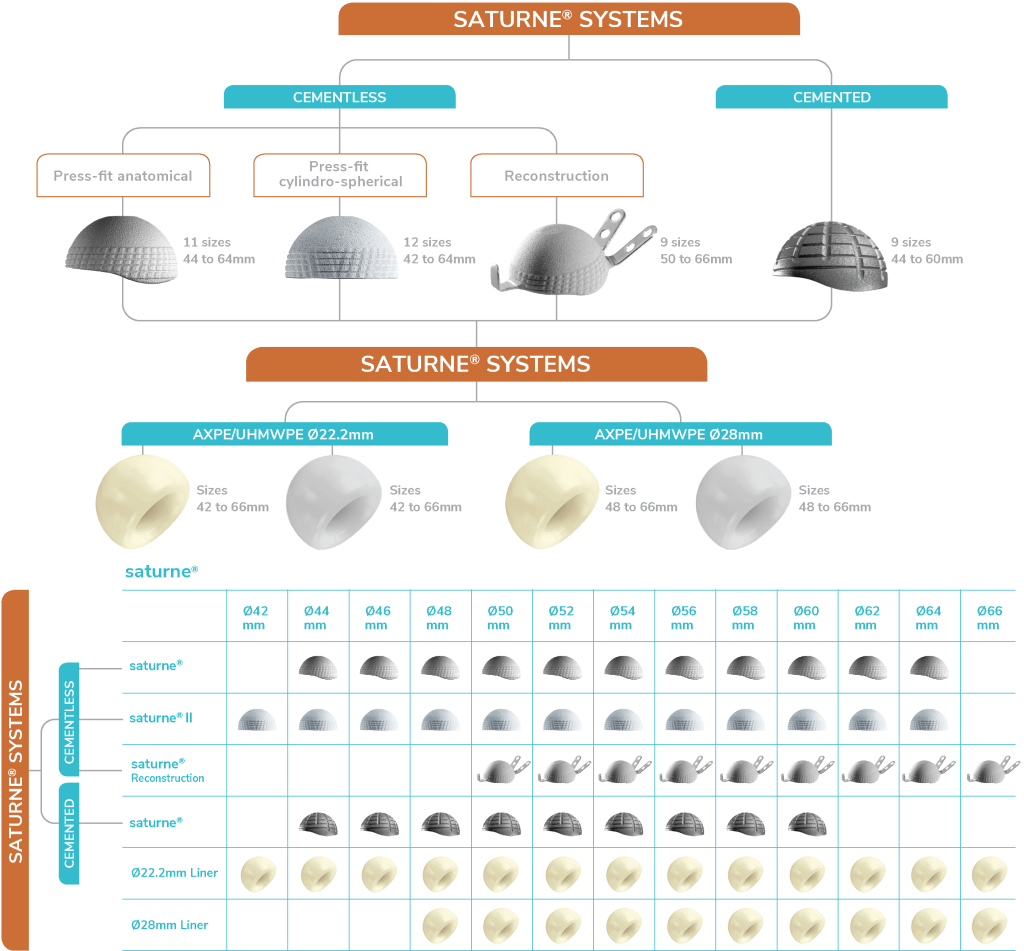
Discover the Saturne Monoblock Dual Mobility Range

SATURNE®
Cementless
SATURNE® II
Cementless

SATURNE®
Cemented

SATURNE®
Reconstruction
Customer Service and Logistics
E. customerservice@amplitude-ortho.com.au
T. 08 8297 9901
38 Payneham Road
Stepney, SA, 5069
Australia
Sales and Marketing
E. marketing@amplitude-ortho.com.au
Suite 6, The Village, 3 Julius Avenue
North Ryde, NSW, 2113
Australia